CLINICAL STUDIES
Kamari Pharma is well positioned to treat extremely debilitating rare skin diseases. We develop
effective disease modifying treatments for patients with great unmet needs.
OLMSTED SYNDROME
Professionals
Patients
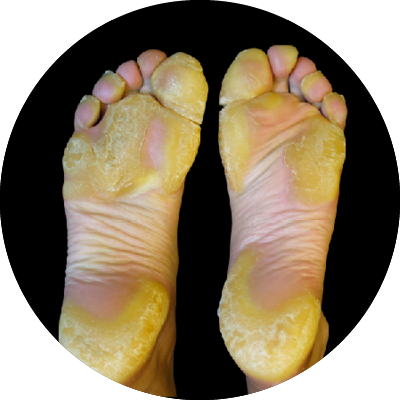
Olmsted Syndrome (OS)
Olmsted Syndrome is an ultra-rare genetic keratinization disorder caused by gain-of-function mutations in the TRPV3 ion channel. Patients present with severe, painful palmoplantar keratoderma, periorificial keratotic plaques, alopecia, and often severe, debilitating pain and itch. OS has significant unmet medical need, with no approved targeted therapies available today.
KM-023 is a first-in-class oral, selective TRPV3 inhibitor designed to target the direct underlying cause of OS. By normalizing calcium signaling in keratinocytes, KM-023 has the potential to reduce hyperkeratosis, alleviate pain, and improve skin inflammation.
Kamari Pharma has initiated a Phase I clinical trial in France to evaluate the safety, tolerability, and pharmacokinetics of KM-023 in healthy volunteers. Following successful completion, Kamari plans to start the first-in-patient clinical study in late 2025 to evaluate safety, tolerability, and efficacy of KM-023 in Olmsted Syndrome patients.
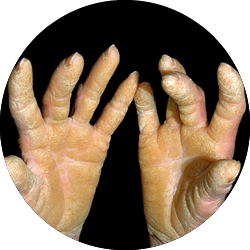
Olmsted Syndrome
Olmsted Syndrome (OS) is a very rare genetic skin disorder caused by changes (mutations) in the TRPV3 gene. These changes lead to an abnormal activation of the TRPV3 channel in skin cells, which can cause painful thickening of the skin on the palms and soles, as well as plaques around the eyes, mouth, and other areas. People living with OS often experience severe pain, itching, and inflammation, with a major impact on quality of life. There are currently no approved treatments that directly target the cause of the disease.
KM-023 is an oral treatment in development by Kamari Pharma. It works by blocking the overactive TRPV3 channel, aiming to reduce skin thickening, pain, and inflammation.
Kamari Pharma has started a Phase I clinical study in France to test the safety of KM-023 in healthy volunteers. Following this study, the company plans to begin the first clinical trial in patients with OS in late 2025.
