CLINICAL STUDIES
Kamari Pharma is well positioned to treat extremely debilitating rare skin diseases. We develop
effective disease modifying treatments for patients with great unmet needs.
PALMOPLANTAR KERATODERMAS
Professionals
Patients
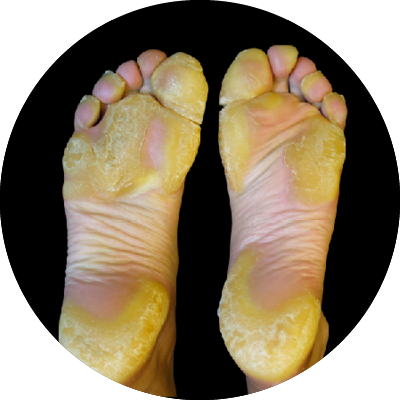
Palmoplantar Keratodermas
The Palmoplantar Keratodermas (PPKs), are a group of rare skin conditions that result from various mutations in several epidermal genes. Mild to moderate PPK’s are characterized by thickening of the skin on the palms and soles leading to great discomfort, severe itch and pain. The PPKs include diseases such as Punctuate PPK type 1 (PPPK1), Pachyonychia Congenita (PC) and Mal de Meleda, all of which have significant unmet medical need.
KM-001 is a novel pharmaceutical topical cream for the treatment of PPPK1 and PC. The Active ingredient is a TRPV3 inhibitor. The TRPV3 ion channel is abundant in the skin’s Keratinocytes and coupled to EGFR. Thus, it may be involved in the mechanism leading to keratodermas development which consist of EGFR excessive activation, like PPPK1 and PC (Wang and Chang 2003; Chen, Wang, and Chang 2007)
Kamari Pharma has completed a phase IB clinical study in PC and PPPK type-1 patients
These are open label clinical studies to assess the safety and efficacy of KM-001 topical cream in 1% strength, in PPPK1 and PC patients. These Phase 1b studies are a Proof-of-concept studies, to evaluate the effectiveness of KM-001 in the treatment of PC and PPPK1 of adult patients.
The studies enrolled patients in Israel and the UK.
See additional details and contact in:
Israel – NCT05435638
UK – NCT05956314
PC Project: Clinical studies
photograph courtesy of the PC Project

Palmoplantar Keratodermas
The Palmoplantar Keratodermas (PPKs), are a group of rare skin conditions that result from various mutations in several epidermal genes. Mild to moderate PPK’s are characterized by thickening of the skin on the palms and soles leading to great discomfort, severe itch and pain. The PPKs include diseases such as Punctuate PPK type 1 (PPPK1), Pachyonychia Congenita (PC) and Mal de Meleda, all of which have significant unmet medical need.
KM-001 is a novel pharmaceutical topical cream for the treatment of PPPK1 and PC.
Kamari Pharma has completed a phase IB clinical study in PC and PPPK type-1 patients
These are open label clinical studies to assess the safety and efficacy of KM-001 topical cream in 1% strength, in PPPK1 and PC patients. These Phase 1b studies are a Proof-of-concept studies, to evaluate the effectiveness of KM-001 in the treatment of PC and PPPK1 of adult patients.
The studies enrolled patients in Israel and the UK.
See additional details and contact in:
Israel – NCT05435638
UK – NCT05956314
PC Project: Clinical studies
